22+ Calculate Atoms To Moles
We assume you are converting between atom and mole. Conversion of moles to atoms The mole is the unit of measurement for amount of substance in the International System of Units SI.

Open Shell Variant Of The London Dispersion Corrected Hartree Fock Method Hfld For The Quantification And Analysis Of Noncovalent Interaction Energies Journal Of Chemical Theory And Computation
The answer is 60221415E23.

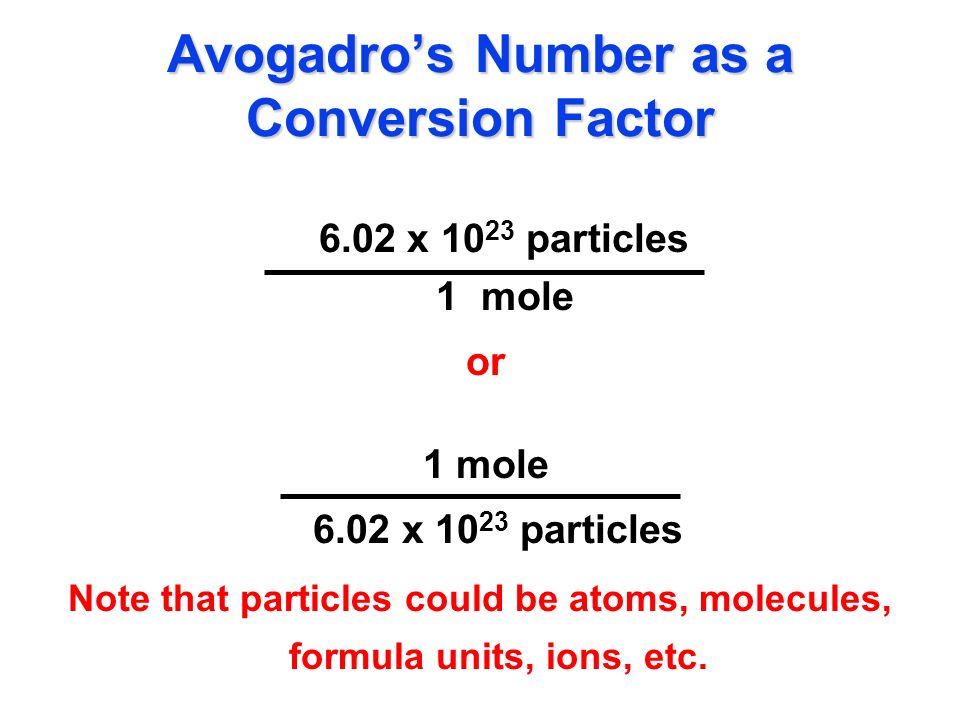
. II 1 mole of a substance has 602 10 X 23 particles or units. Moles of Carbon 472 1024 1mole6022140857 x 1023. Mass of the material Number of Moles of the material Press the calculate button.
By estimating the size of atoms and taking volume measurements of 1 mole samples scientists can estimate that 1 mole 6022 10 23. Converting moles of a substance to atoms requires a conversion factor of Avogadros. It is defined as exactly 60221407610 23 particles.
You can view more details on each measurement unit. Find the Molar Mass of the Formula. Have you eaten a.
Simply put one mole of a substance atoms molecules ions etc. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. Atoms Moles x Avogadros number Alan.
NaCl Na Cl NaCl 229898. To convert atoms to moles you need to use a calculator. There is no conversion formula between the two.
The answer is 60221415E23. In this video well learn to how to determine the number of atoms in one mole of a substance. Select Molecular Weight from the drop down menu titled Calculate.
Yes we can calculate the number of atoms using the atoms to moles formula because both these values are interconvertible. To use the formula multiply the number of moles by Avogadros. Then the number of moles of the substance must be converted to atoms.
11 rows 1 mole 6022140857 x 1023 atoms. If your sample is made of one element like copper. First enter the number of atoms you have.
So how many things are in a mole. To use the calculator simply enter the number of moles and click calculate The answer will be in atoms. Think of moles like a dozen.
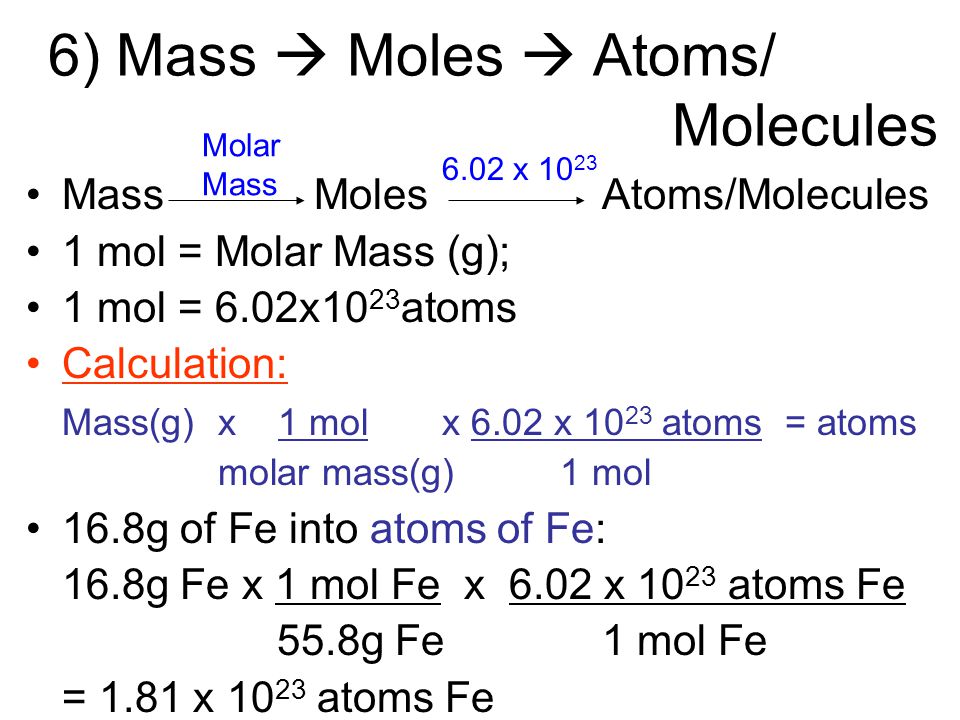
I 1 mole of a substance equals it atomic or molecular weight. 472 10 24 atoms C 1 mol C 602 10 23 atoms C. One conversion factor will allow us to convert from the number of C atoms to moles of C atoms.
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. Then press the button that says Convert The calculator will then show. We assume you are converting between atom and mole.
You can view more details on each measurement unit. How many atoms in 1 mole. To Calculate Molar Mass.
Converting Moles to Grams and Vice Versa Grams and moles are two units that express the amount of matter in a sample. The SI base unit for amount of. Find a periodic table of elements to find the molar mass of your sample.

Atoms To Moles Calculator Dbcalculator Com

Calculate Moles Molecules And Atom Just In 5 Minute Youtube

Chemical Quantities Ch Ppt Download

I4j6baciq2eblm
How Many Atoms Are There In 0 45 Mol Of Aluminium Socratic

Hfld A Nonempirical London Dispersion Corrected Hartree Fock Method For The Quantification And Analysis Of Noncovalent Interaction Energies Of Large Molecular Systems Journal Of Chemical Theory And Computation

Math Physics Chemistry Questions Discussion Lists Dated 2016 10 11

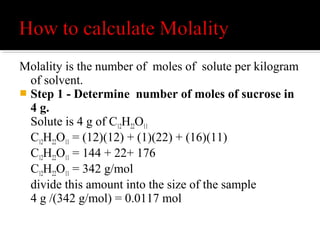
Mole Conversions
:max_bytes(150000):strip_icc()/pipette-pouring-water-on-sugar-cube-in-spoon-663787601-57fb97c23df78c690f799c5c.jpg)
Molarity Example Problem Converting Mass To Moles
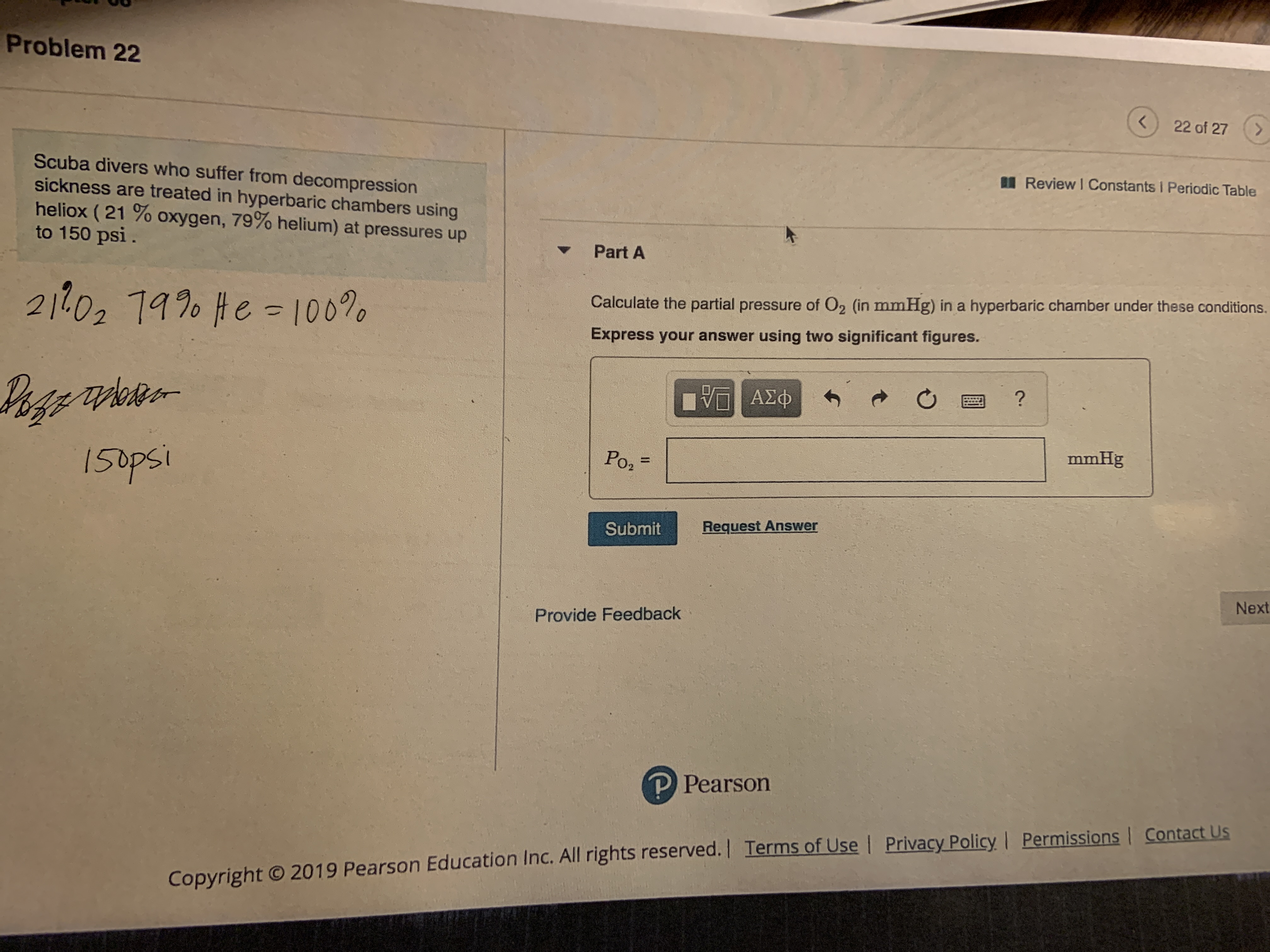
Answered Problem 22 22 Of 27 Scuba Divers Who Bartleby
Moles To Atoms Formula Using Avogadro S Number Video Lesson Transcript Study Com
Calculations Based On Balanced Equations
Chemical Quantities Ch Ppt Download

Answered The Pressure In Interplanetary Space Is Bartleby

Open Shell Variant Of The London Dispersion Corrected Hartree Fock Method Hfld For The Quantification And Analysis Of Noncovalent Interaction Energies Journal Of Chemical Theory And Computation

Moles Part 1 Particles Aim Ce3 How Many Different Measures Is A Mole Ppt Download
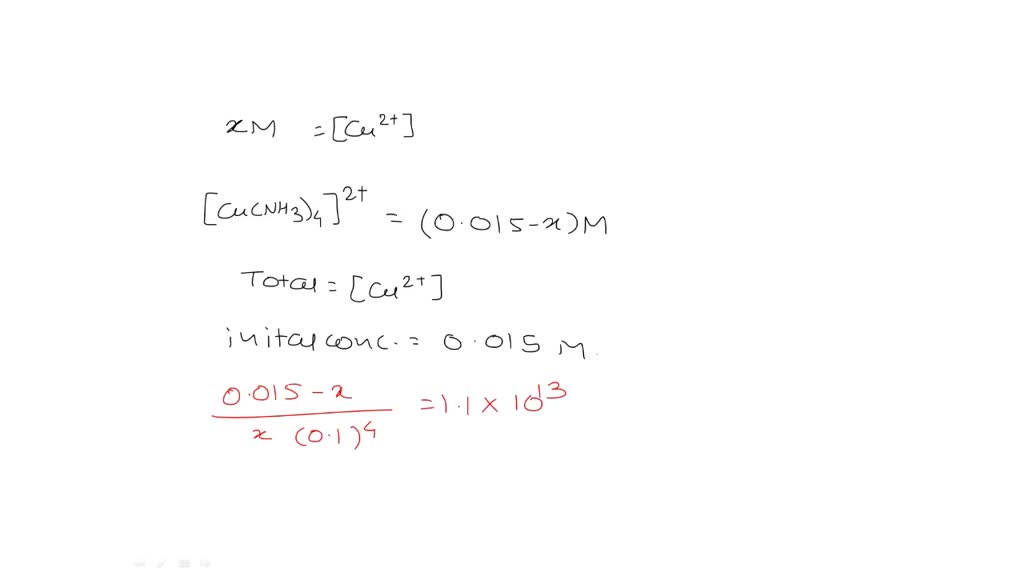
Solved Part 1 Copper Ii Forms A Complex With Four Molecules Of Ammonia According To The Following Cu2 Aq Nh3 Aq Cu Nh3 2 Aq K1 1 9 104 Cu Nh3 2 Aq Nh3 Aq Cu Nh3 22 Aq K2